About iFraP
The iFraP study is developing and testing a package of resources to support clinicians and patients make informed decisions about osteoporosis medicines to improve patient-centred care.
For people with osteoporosis, fragility fractures (occurring from events that would not normally result in a broken bone) can result in significant disability, pain, and increased risk of loss of independence. Medicines are available to maintain bone density and reduce the chance of a fragility fractures for people with osteoporosis. However, many people who experience a fragility fracture do not start the recommended osteoporosis medicines. Of those who start treatment, fewer than half continue taking treatment for more than one year. If we increase the number of people with osteoporosis that start, and continue taking osteoporosis medications we will lower the number of future fractures, and associated negative outcomes (e.g. significant disability).
It is important that we support healthcare professionals to discuss medicines with the patient and to explain the risks and benefits of medications to help patients to make a decision about osteoporosis that is right for them. One way to support this is to use a ‘decision-support tool’.
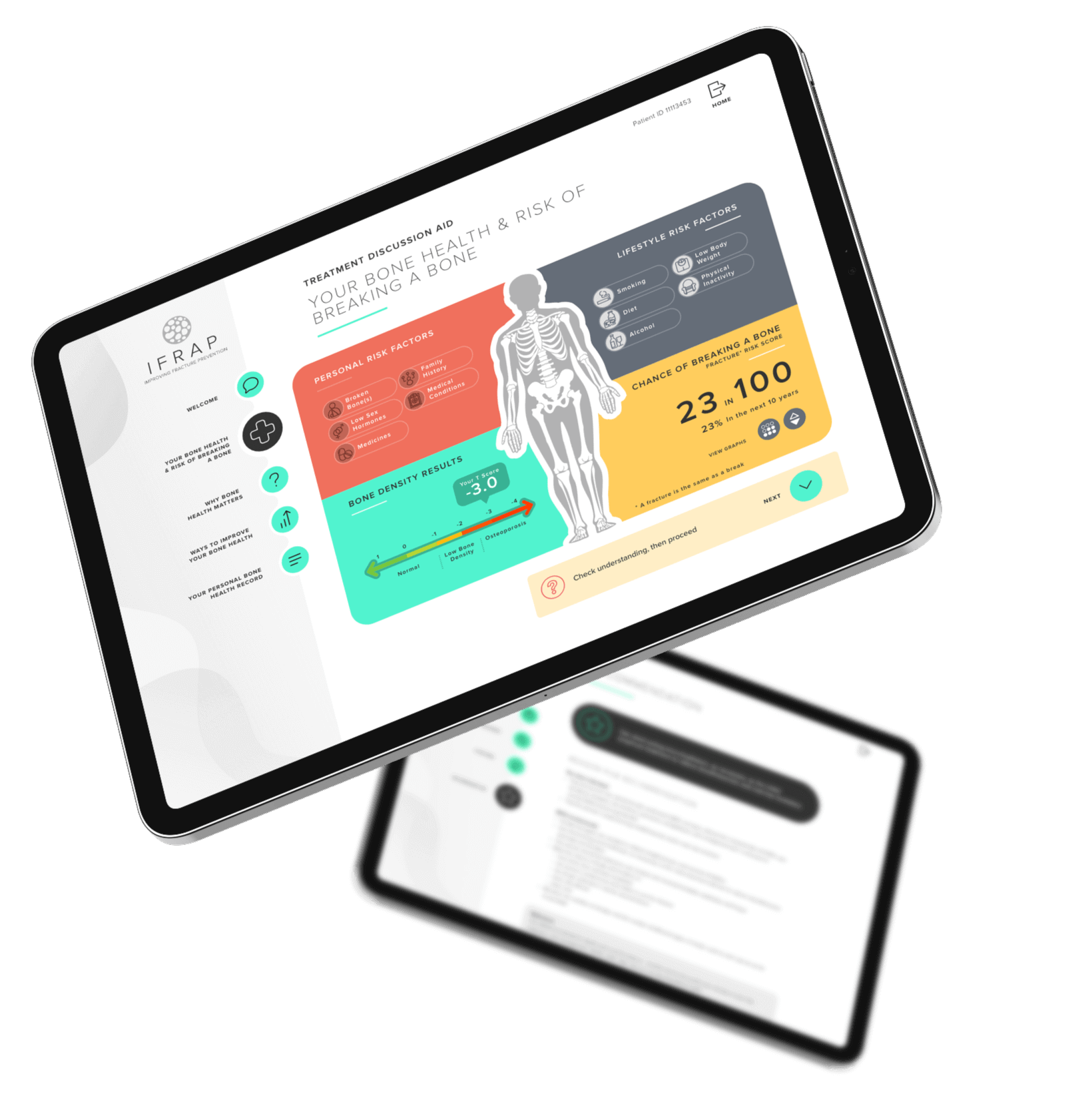
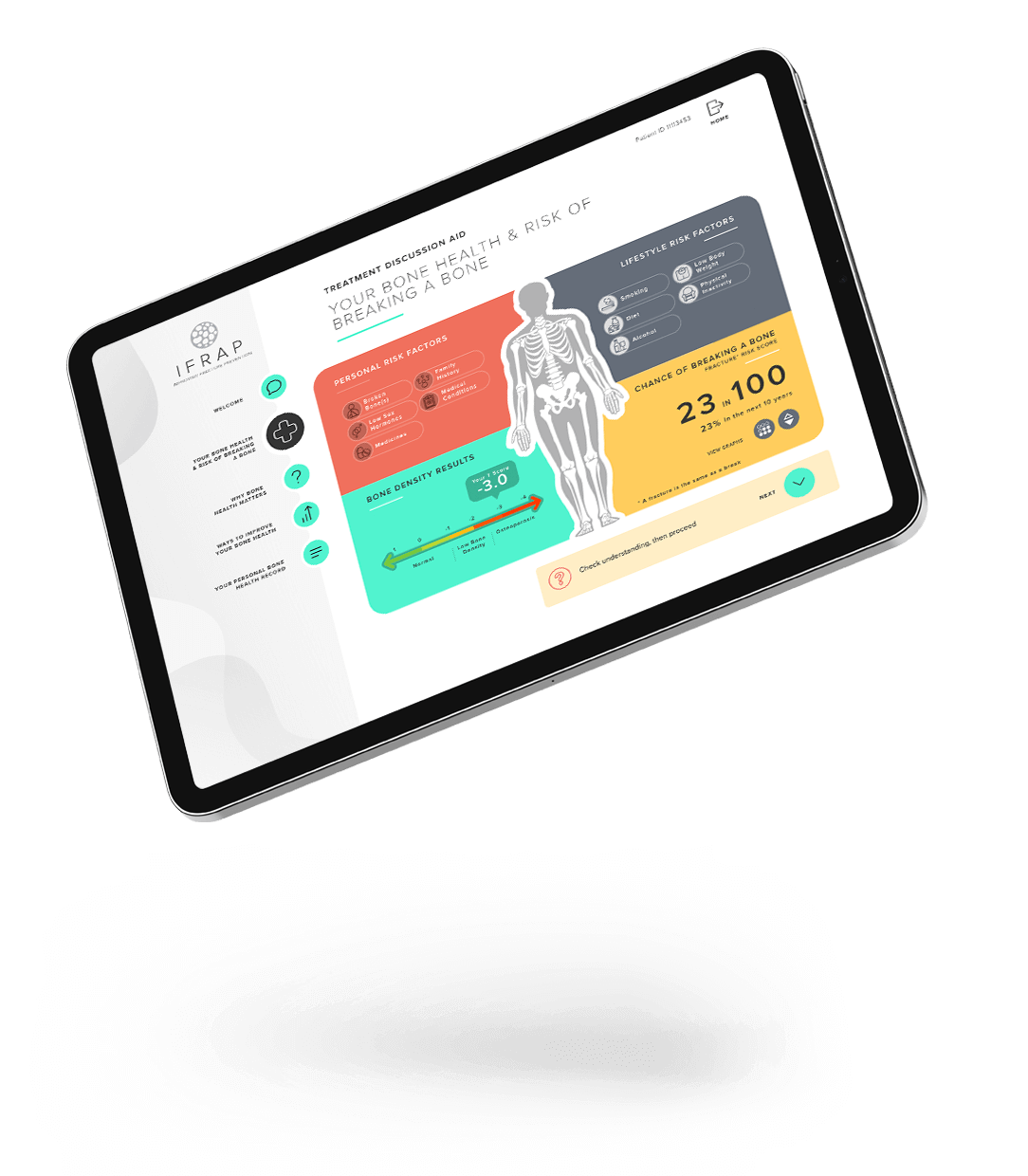
‘Decision-support tools’ can support healthcare professionals to know when to suggest treatments and can help healthcare professionals and patients talk together about risk and to make decisions, for instance about starting a new medicine. The iFraP study developed a computer based ‘tool’ that can be used in appointments after someone has had a fragility fracture. We have also developed training for healthcare professionals to help them use the tool and to ensure that the information they are giving is understandable, that they address patient concerns, and that they give clear, consistent information during the appointment. Further information on how the iFraP resources were developed is outlined below.
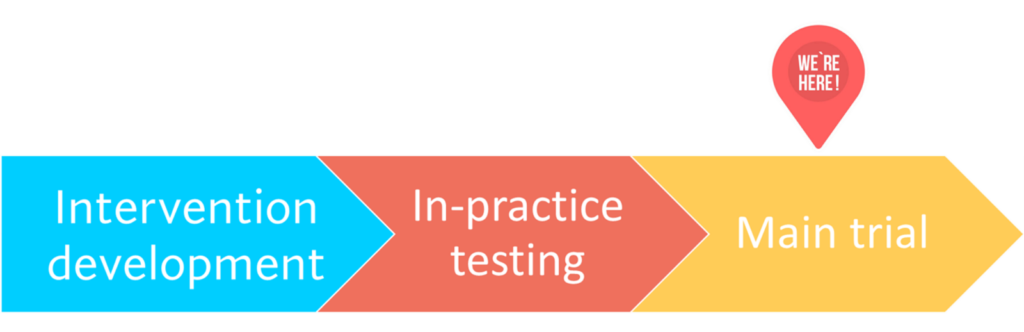
The iFraP study involves three phases:
Intervention development
In-practice testing
Main trial
Click on the video to learn more about how we developed the iFraP resources
Now that we have developed the iFraP resources, we will now complete a trial to test the iFraP intervention in Fracture Liaison Services on a much larger scale. The trial will look at whether the iFraP intervention makes decision making about osteoporosis medicines easier, and whether it is cost-effective, acceptable and practical to deliver. Patients taking part in the trial will be allocated to receive one of two options: usual NHS care or the iFraP intervention.
Paskins Z, Torres Roldan VD, Hawarden AW, Bullock L, Meritxell Urtecho S, Torres GF, Morera L, Espinoza Suarez NR, Worrall A, Blackburn S, Chapman S, Jinks C, Brito JP. 2020. Quality and effectiveness of osteoporosis treatment decision aids: a systematic review and environmental scan. Osteoporos Int, vol. 31(10), 1837-1851. https://doi.org/10.1007/s00198-020-05479-w
Hawarden A, Jinks C, Mahmood W, Bullock L, Blackburn S, Gwilym S, Paskins Z. 2020. Public priorities for osteoporosis and fracture research: results from a focus group study. Arch Osteoporos, vol. 15(1), 89. https://doi.org/10.1007%2Fs11657-020-00766-9
Paskins Z, Jinks C, Mahmood W, Jayakumar P, Sangan CB, Belcher J, Gwilym S. 2017. Public priorities for osteoporosis and fracture research: results from a general population survey. Arch Osteoporos, vol. 12(1), 45. https://doi.org/10.1007/s11657-017-0340-5
Raybould G, Babatunde O, Evans AL, Jordan JL, Paskins Z. 2018. Expressed information needs of patients with osteoporosis and/or fragility fractures: a systematic review. Arch Osteoporos, vol. 13(1), 55. https://doi.org/10.1007/s11657-018-0470-4
Crawford-Manning F, Greenall C, Hawarden A, Bullock L, Leyland S, Jinks C, Protheroe J, Paskins Z. 2021. Evaluation of quality and readability of online patient information on osteoporosis and osteoporosis drug treatment and recommendations for improvement. Osteoporos Int. vol. 32(8):1567-1584. https://doi.org/10.1007/s00198-020-05800-7
To learn more about how we developed the iFraP resources
Paskins Z, Bullock L, Crawford-Manning F, Cottrell E, Fleming J, Leyland S, Edwards JJ, Clark E, Thomas S, Chapman SR, Ryan S, Lefroy JE, Gidlow CJ, Iglesias C, Protheroe J, Horne R, O’Neill TW, Mallen C, Jinks C. 2021. Improving uptake of Fracture Prevention drug treatments: a protocol for Development of a consultation intervention (iFraP-D). BMJ Open. Vol. 18;11(8). https://doi.org/10.1136/bmjopen-2021-048811


This study has been reviewed and given favourable opinion by East of Scotland Research Ethics Service (reference: 22/ES/0038).